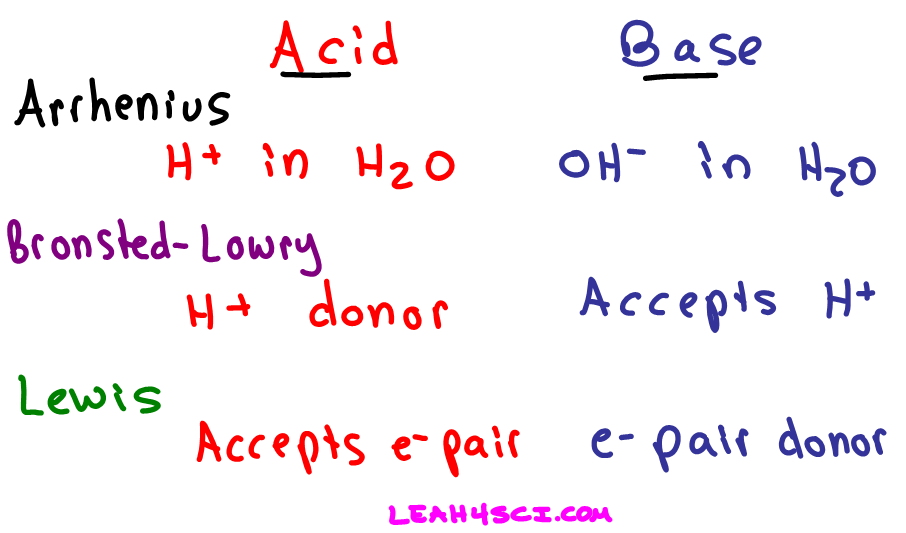Acids and Bases 3 definitions for acids and bases – Arrhenius – Bronsted-Lowry – Lewis Must be in solution – Most often dissolved in water (aqueous) Inorganic. - ppt download

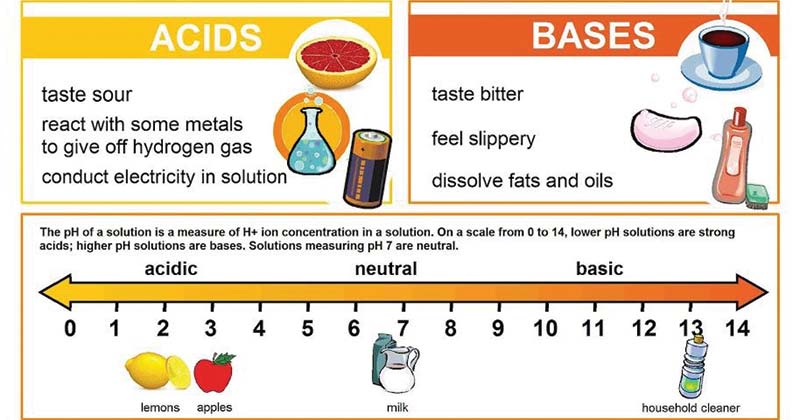
Acids and Bases. Different Definitions of Acids and Bases Arrhenius definitions for aqueous solutions. acid: acid: a substance that produces H + (H ppt download

Definitions. Arrhenius Acids and Bases Acids release hydrogen ions in water. Bases release hydroxide ions in water. An acid is a substance that produces. - ppt download
![Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download](https://images.slideplayer.com/20/6056284/slides/slide_2.jpg)
Acids and Bases Topics to be covered: Definitions of acids and bases; Bronsted's conjugate acid-base pairs concept; Determination of [H 3 O + ], [OH - - ppt download
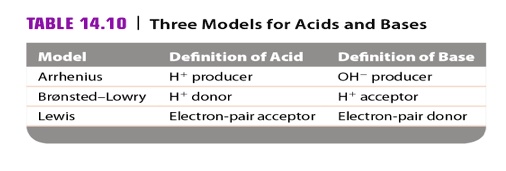
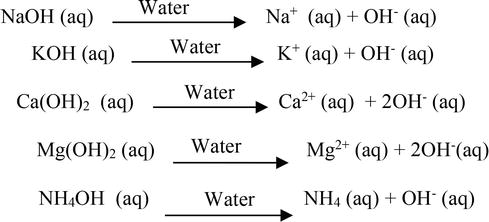
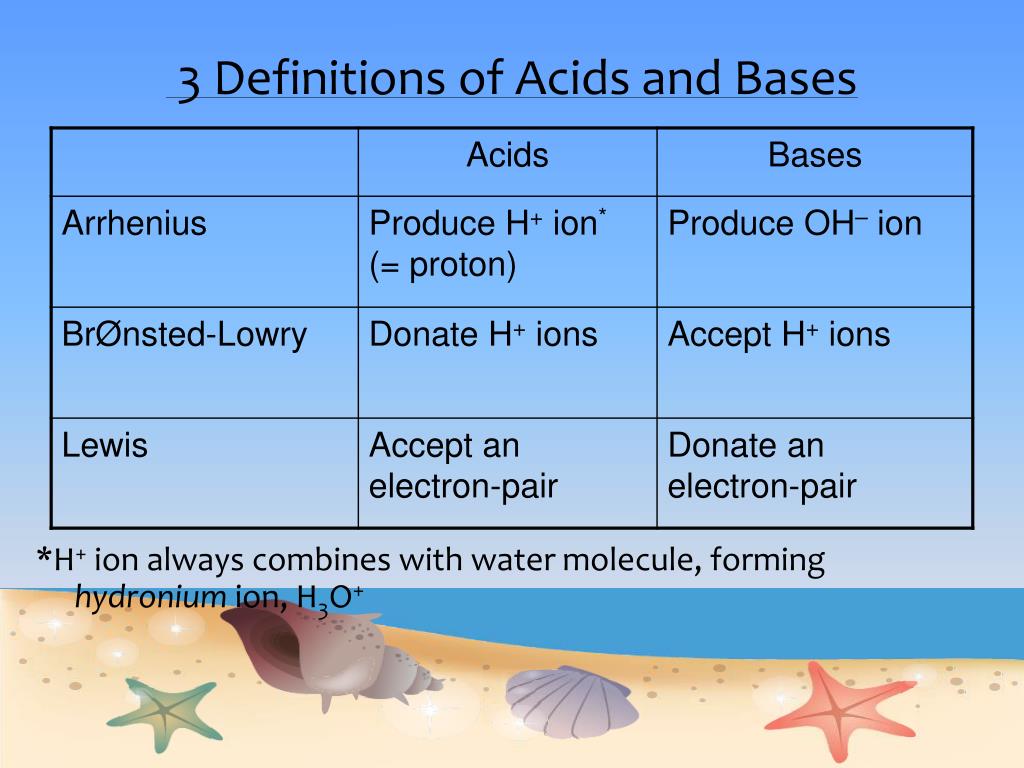
SOLVED: TABLE 14.10 Three Models for Acids and Bases Model Arrhenius Bronsted Lowry Lewis Delinition of Acid producer Definition of Base OH producer acceptor Electron-pair donor donor Electron-pair acceptor
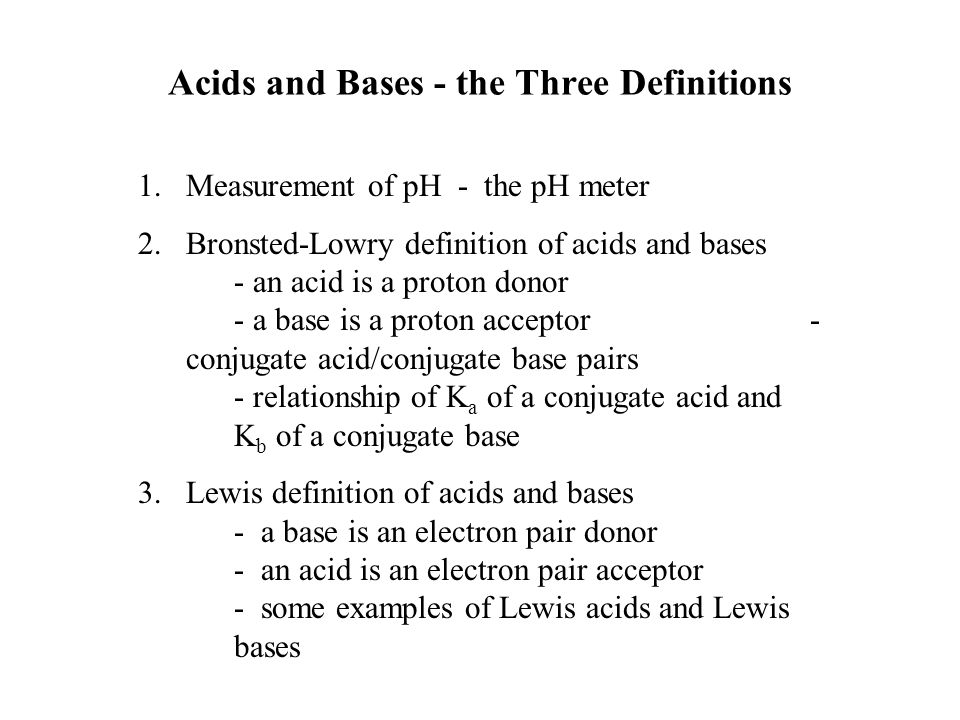
Acids and Bases - the Three Definitions 1.Measurement of pH - the pH meter 2.Bronsted-Lowry definition of acids and bases - an acid is a proton donor - - ppt download
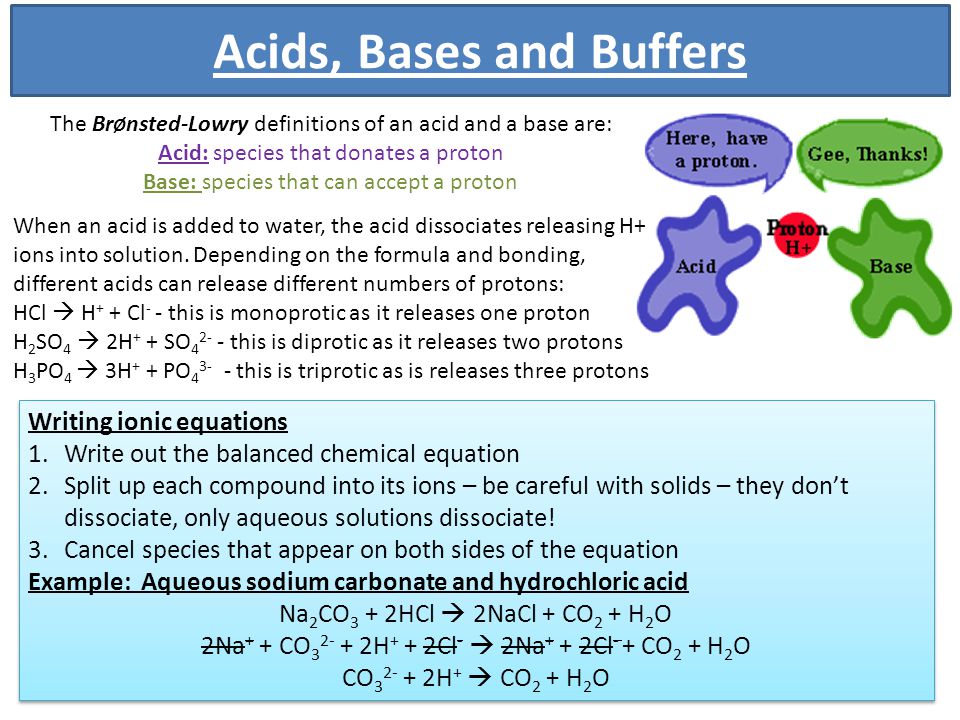
Acids, Bases and Buffers The Br Ø nsted-Lowry definitions of an acid and a base are: Acid: species that donates a proton Base: species that can accept. - ppt download

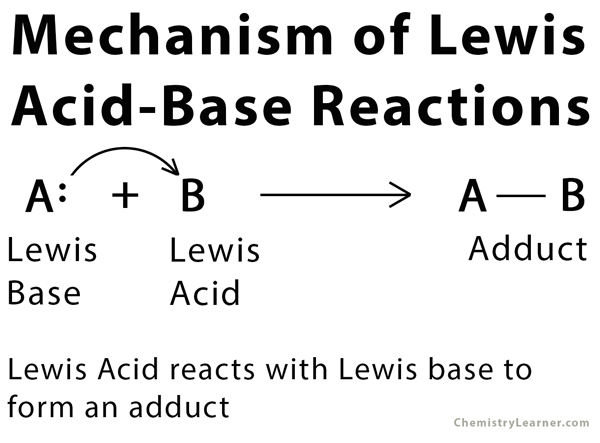
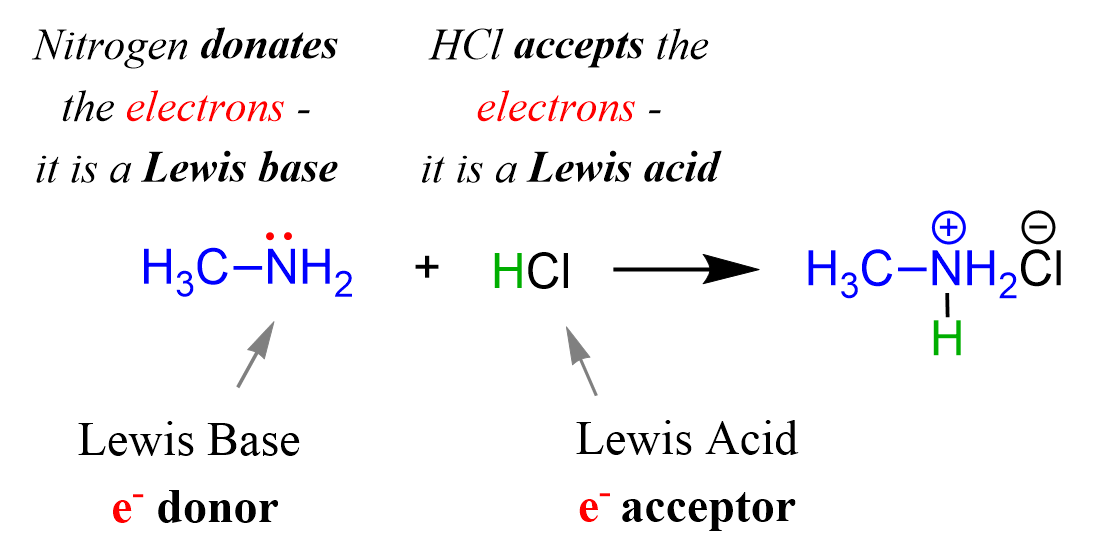
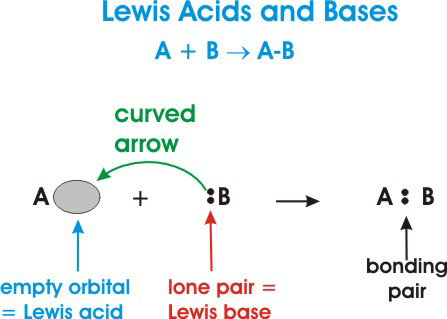
Lewis Acids and Bases - Definition,Properties, Examples, Reactions, Uses, Applications of Lewis acids and Bases.